
Rumors, Half-Truths and Truths about Temperature and pH

A few months ago, we received a call from a customer utilizing one of our differential probes and a controller. Embedded in the probe is a thermistor that measures temperature. The controller corrects for its effect on pH. He calibrated his probe in the comfort of his control room but, when he put the probe in his warm process, the pH read low by 0.2 pH units. Clearly temperature compensation was not working. He was ready to demand a refund. When I learned that his process was approximately 40 °C I explained that his probe was working fine. It was his interpretation of the reading that was wrong.
Few issues are as confusing or multifaceted as the effect of temperature on pH. Most users understand that temperature changes the pH value and that temperature compensation corrects for this effect. But they’re not quite sure how it works. And most don’t understand that there is an effect of temperature that is very real and yet another that can cause error.
This white paper will attempt to set the record straight so that the user can get real, actionable information from his or her pH analyzer.
The largest temperature dependence effect is not real.
First let’s get one thing straight. For a pH probe “7” is the “new zero.” A probe in pure water, at 25 °C, measures a pH of 7 and outputs a voltage close to 0 volts. As the pH drops the probe output increases and, as the pH increases, the output decreases by dropping into negative territory. The output voltage varies with pH according to the singularly most important mathematical formula in all of electrochemistry, the Nernst equation, here adapted for the pH probe.
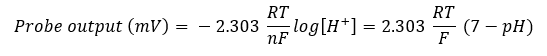
If we multiply the constants R and F by a temperature of 298 K (equivalent to 25 °C) then we get the one number that every pH user should know—59.16 mV/pH. This means that, for every change of 1 pH unit, the probe output changes by 59.16 mV. The change is positive as the pH decreases and negative as it increases. A pH analyzer is nothing more than a very good (i.e. high impedance) voltmeter. So a solution with a pH of 4 will ideally output a probe voltage of 3 x 59.16 mV, or 177.48 mV.
BUT this is only true at 25 °C. At any other temperature the Nernst equation gives a different voltage. Fortunately, that voltage is just as easy to calculate since the probe output is proportional to temperature. Rather than recalculate the Nernst equation for every temperature we recast it in the following form:
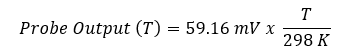
With this approach we start with 59.16 mV/pH and make a correction for the actual temperature. This is exactly what temperature compensation does.
However, immerse a probe in pure water, heat the water and watch the pH drop. Temperature compensation is still working just fine. The pH drops because…
… Neutral is NOT pH 7 if the temperature is not 25 °C.
We never give it a second thought: Water at pH 7 is neutral water—neither acidic nor basic. But that’s only true at one temperature — 25 °C.
In any sample of water a small fraction of water molecules break apart—dissociate—to form H+ and OH– ions:
![]()
In pure water the two concentrations are necessarily equal. The “7” value comes from the concentration H+ and OH– ions in water in 25 °C. It’s 10-7 moles/liter. At higher temperatures a greater fraction of water molecules dissociate.
At 40 °C that fraction is 10-6.7 so the pH of neutral water is 6.7. 10 °C the pH of neutral water is 7.3.
Many guides advise users to calibrate at the temperature of the process. This is sound advice but it has nothing to do with the temperature dependence of pH. The reason is because….
… Even perfect probes have zero offsets that change with temperature.
The offset is probe output when the probe is in neutral solution, e.g. pH 7 at 25 °C. Several potentials contribute to the offset. The two major sources are:
- The difference in potential between the gel layer on inside of the glass membrane —the side that is bathed in constant pH 7 solution—and the gel layer on the outside of the glass—the side exposed to the process. Even when the process is neutral differences in the gel layer produce a non-zero offset
- The diffusion potential at the liquid junction. The differing diffusion rates of the ions in the reference chamber—usually K+ and Cl—create a charge separation between the two and a potential. This potential is the one that exists even in an ideal probe when the probe is ideal and the offset created by the gel layer asymmetry is zero. The potential is on the order of 2 mV.
Both of these contributions are temperature dependent. So it makes sense that, to minimize changes in the offset that are temperature related, that one should calibrate and measure at the same temperature.
The fact that the offset is a function of temperature casts doubt on one point frequently made in the literature…
…The isothermal intersection point is not fixed.
The isothermal intersection point is the value of the pH and millivolt output of a pH probe at which two calibration curves for two temperatures intersect. If the offset were zero then all calibration curves for different temperatures would intersect at pH 7 and 0 mV. If the offset were non-zero but constant, then all calibration curves would intersect at one point and the isothermal intersection point would be a constant. Since the offset does depend on temperature there is no one unique isothermal intersection point. In the figure below are three calibration curves. This figure is not from real data and is meant to be illustrative only. The three curves intersect in the same vicinity but not exactly at the same point.
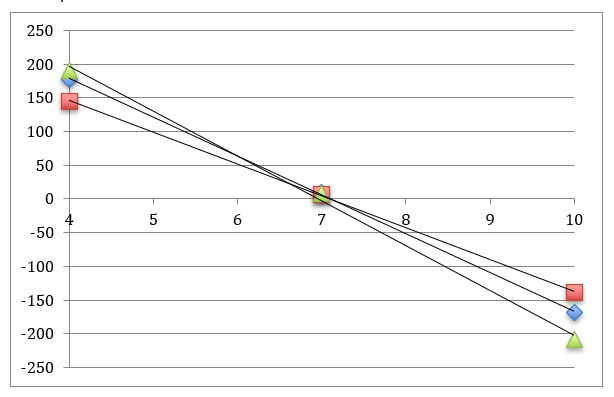
Measuring and calibrating at the same temperature will insure that you determine the correct offset. However, be forewarned that you can still mess up your calibration for the simple reason that…
… The pH of calibration solutions changes with temperature.
Since water changes its pH with temperature there is no reason why buffer solutions wouldn’t be subject to the same issue. In fact every dissolved compound that affects pH does so in a way that is temperature dependent. Buffers are solutions of weak acids, which partially dissociate into H+ cations and salt anions. Most buffers decrease in pH for the same reason water does, i.e. that as the buffer dissociates the concentration of H+ ions increases. This is not always the case.
Fortunately, there are tables and mathematical formulas that give the dependence of the pH buffer actual pH on temperature. At 40 °C the pH of the 4, 7 and 10 buffer are 4.03, 6.97 and 9.85. I’ve included a table with pH values for the three common calibration standards at several temperatures. Clearly pH 4 buffer is an exception to the explanation just offered.
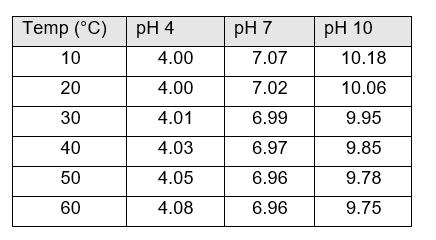
The guideline above—calibrating and measuring at the same temperature—will NOT remove the temperature dependence of buffer solutions. You still must input the correct pH of your calibration standard into the analyzer performing the calibration.
The information in this white paper is explained in greater detail in Mark Spencer’s upcoming book on instrumentation that will be published later this year by AWWA. You can ask him questions or request to be notified when the book is in publication by sending Mark an email at mspencer@wateranalytics.net.

